Multiple Choice
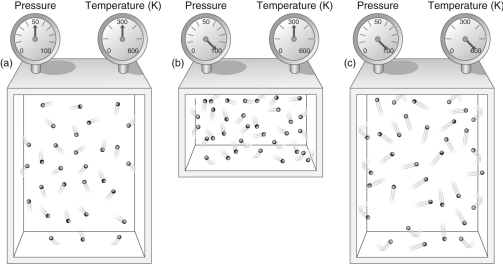 Figure 1
Figure 1
-According to Figure 1, what effect does doubling the temperature of a gas while keeping its volume constant have on the pressure of the gas?
A) The pressure doubles.
B) The pressure is cut in half.
C) The pressure remains the same.
D) The volume quadruples.
E) The volume is cut to one-third its original value.
Correct Answer:

Verified
Correct Answer:
Verified
Q65: All the terrestrial planets have atmospheres as
Q66: According to the figure, about how long
Q67: The fraction of oxygen in the Earth's
Q68: Which molecule moves with the fastest average
Q69: According to the Ideal Gas law, if
Q71: The major chemical component of the air
Q72: Venus's surface temperature is fairly uniform from
Q73: If it were not for the greenhouse
Q74: List the three planets shown in the
Q75: Humans cannot survive on the surface of