Essay
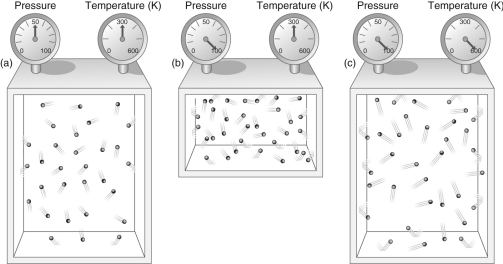 Figure 2
Figure 2
-According to Figure 2, what effect does doubling the temperature of a gas while keeping its volume constant have on the pressure of the gas?
Correct Answer:

Verified
Because the ideal gas law says...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Because the ideal gas law says...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q41: According to the figure shown below, as
Q43: By examining the images shown below, what
Q45: A gas eventually will escape from a
Q47: Venus rotates so rapidly that the dominant
Q48: When the Martian summer occurs and the
Q49: According to the figure shown below, the
Q51: Venus and Earth probably formed with similar
Q66: The main greenhouse gases in the atmosphere
Q67: Hurricanes are powered by<br>A) Hadley circulation.<br>B) the
Q87: Most of Earth's present-day atmosphere comes from