Essay
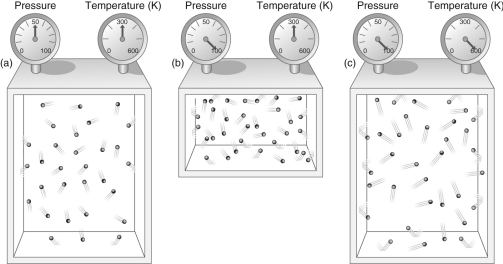 Figure 2
Figure 2
-According to Figure 2, what effect does doubling the pressure on a gas while keeping its temperature constant have on the volume of the gas?
Correct Answer:

Verified
Because the ideal gas law says...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Because the ideal gas law says...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q6: When frozen water on the surface of
Q35: The global winds on Earth are the
Q61: Would water molecules in Venus's atmosphere, whose
Q83: When learning about light, we predicted that
Q85: Based solely on mass and distance from
Q86: According to the way the layers of
Q87: Over the last century, why has the
Q89: If the carbon dioxide in Earth's rocks
Q91: What is the reason Mercury has so
Q92: If there are 10<sup>4</sup> kg of air