Essay
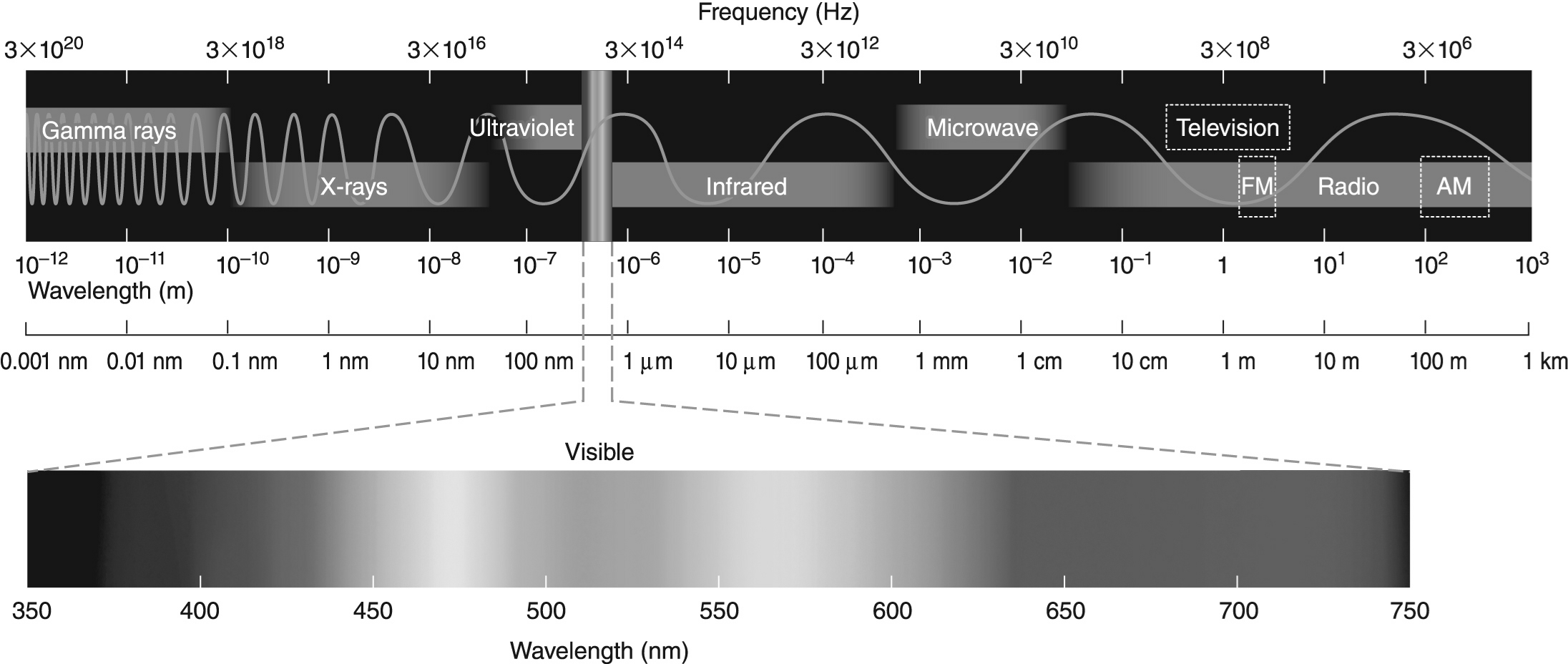 Figure 1
Figure 1
-The difference in energy between the n = 2 and n = 1 electronic energy levels in the hydrogen atom is 1.6 * 10-18 J. If an electron moves from the n = 1 level to the n = 2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown above to answer this question.
Correct Answer:

Verified
The n = 2 energy level is higher than th...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q20: Which formula denotes how the speed
Q37: Why do some stars in the sky
Q38: Einstein showed that the _ could be
Q39: Cooler objects radiate more of their total
Q41: Saying that something is quantized means that
Q43: What does it mean to say that
Q44: What two factors control a planet's surface
Q45: The average red giant in the night
Q46: Why is an iron atom a different
Q89: Name four physical properties of an object