Multiple Choice
If an average hydrogen atom in Earth's atmosphere has a velocity of 2.5 km/s,what would be the average velocity of an 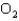 molecule in the Earth's atmosphere? Note that the atomic mass of an oxygen atom is 16 times that of a hydrogen atom.
molecule in the Earth's atmosphere? Note that the atomic mass of an oxygen atom is 16 times that of a hydrogen atom.
A) 0.16 km/s
B) 2.5 km/s
C) 0.62 km/s
D) 0.44 km/s
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Mars' atmosphere is mostly composed of carbon
Q2: If water vapor was released from Venus'
Q5: If you found absorption arising from _
Q6: Hurricanes are powered by<br>A) Hadley circulation.<br>B) the
Q7: Which of the following processes did NOT
Q8: The major component of the air we
Q9: Without the ozone layer,life on Earth would
Q10: A gas eventually will escape from a
Q11: The greenhouse effect raises Earth's surface temperature
Q87: Most of Earth's present-day atmosphere comes from