Multiple Choice
The 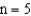 electronic energy level in a hydrogen atom is
electronic energy level in a hydrogen atom is 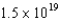 J higher than the
J higher than the 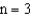 level.If an electron moves from the
level.If an electron moves from the 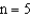 level to the
level to the 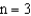 level then a photon of wavelength
level then a photon of wavelength
A) 1.3 nm,which is in the ultraviolet region,is emitted.
B) 1.3 nm,which is in the ultraviolet region,is absorbed.
C) 1,300 nm,which is in the infrared region,is absorbed.
D) 1,300 nm,which is in the infrared region,is emitted
Correct Answer:

Verified
Correct Answer:
Verified
Q10: If an object is moving toward you,
Q34: The luminosity of an object is independent
Q60: Which of the following photons carry the
Q62: Einstein showed that the _ could be
Q63: The Kelvin temperature scale is used in
Q64: At what wavelength does your body radiate
Q67: How do the wavelength and frequency of
Q68: In our solar system,a planet's temperature depends
Q69: If a street lamp at a distance
Q73: Suppose you observe a star emitting a