Essay
The difference in energy between the 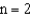 and
and 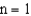 electronic energy levels in the hydrogen atom is
electronic energy levels in the hydrogen atom is 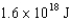 .If an electron moves from the
.If an electron moves from the 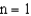 level to the
level to the 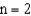 level will a photon be emitted or absorbed? What will its energy be,and what type of electromagnetic radiation is it?
level will a photon be emitted or absorbed? What will its energy be,and what type of electromagnetic radiation is it? 
Correct Answer:

Verified
The  energy level is higher th...
energy level is higher th...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q22: An emission line is produced when an
Q46: A radio photon travels more slowly than
Q47: The average red giant in the night
Q51: Compare and contrast the wavelengths, frequencies, speeds,
Q52: You record the spectrum of a star
Q53: The fact that the speed of light
Q54: The total number of neutrons and protons
Q55: The period of a wave is inversely
Q56: The reason that Earth is hotter than
Q66: A spaceship approaches Earth at 0.9 times