Multiple Choice
On the basis of the kinetic theory of gases, when the absolute temperature is doubled, the average kinetic energy of the gas molecules changes by a factor of
A) 16
B) 2
C) 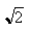
D) 4
E) 0.5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: A collection of oxygen molecules occupies a
Q24: The temperature in January in Winnipeg,Manitoba,has been
Q26: Use the following to answer the question:
Q30: If it is known that two bodies
Q46: A 1 L container contains O<sub>2</sub>
Q46: In a vacuum system,a container is
Q49: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" Which curve correctly
Q50: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6080/.jpg" alt=" The graph shows
Q53: Use the following to answer the question:
Q70: A mass of air is at pressure