Multiple Choice
What is the formal charge on oxygen in the following structure? 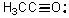
A) +2
B) +1
C) 0
D) -1
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q144: Constitutional isomers differ in the _.
Q145: How many electrons contribute to pi bonds
Q146: Which of the following compounds contain a
Q147: Which of the following is a set
Q148: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt=" is a generalized
Q150: Identify the atomic orbitals in the N-O
Q151: Which molecule would be linear? (In each
Q152: Identify the atomic orbital the lone pair
Q153: An orbital is defined as a region
Q154: When atomic orbitals of opposite phase overlap