Multiple Choice
Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown; however,electrical charges have been omitted deliberately. 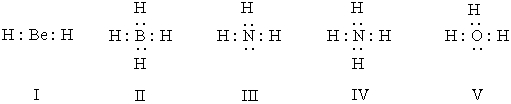 Which of the structures actually bear(s) a positive charge?
Which of the structures actually bear(s) a positive charge?
A) I
B) II
C) III
D) III and V
E) IV and V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of these is a correct electron-dot
Q2: What is the hybridization of the C
Q3: The greatest degree of ionic character is
Q4: What is the hybridization of the N
Q5: How many s-sp<sup>3</sup> bonds are there in
Q7: When the 1s orbitals of two hydrogen
Q8: Which of the structures below would be
Q9: Which of these substances contain both covalent
Q10: Which of the following best describes the
Q11: Which compound is not a constitutional isomer