Multiple Choice
Identify the atomic and/or hybridized orbitals in the C-O sigma bond in acetone. 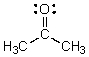
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2p,2p)
E) (2sp,1s)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q86: Which of the following species is/are not
Q87: Which of the following species exhibits resonance
Q88: In which structure(s)below does nitrogen have a
Q89: Draw the Lewis structure of nitroethane CH<sub>3</sub>CH<sub>2</sub>NO<sub>2</sub>,clearly
Q90: There are three fundamental rules that we
Q92: Considering Lewis structures,which of these compounds possesses
Q93: Which of the following species is/are not
Q94: According to molecular orbital theory,in the
Q95: Which of the following species are resonance
Q96: Listed below are electron dot formulas for