Multiple Choice
The bond angle for the C-S-C bonds in the following molecule would be expected to be approximately: 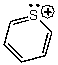
A) 90
B) 109
C) 120
D) 145
E) 180
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: What geometry does the methyl cation,CH<sub>3</sub><sup>+</sup>,have?<br>A)octahedral<br>B)tetrahedral<br>C)trigonal planar<br>D)linear<br>E)trigonal
Q47: What is the formal charge on oxygen
Q48: What is the geometry of the C
Q49: Which of these structures would be a
Q50: Which of the following structures is/are not
Q52: What is the approximate hybridization state of
Q53: Credit for the first synthesis of an
Q54: What bond angle is associated with
Q55: What is the hybridization of the N
Q56: The major resonance contributor to the resonance