Multiple Choice
Which of these compounds would have the highest boiling point?
A) CH3OCH2CH2CH2OCH3
B) CH3CH2OCH2CH2OCH3
C) CH3CH2OCH2OCH2CH3
D) 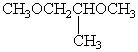
E) HOCH2CH2CH2CH2CH2OH
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q139: The _ is defined as the product
Q140: Hydrocarbons containing carbon-carbon double bonds are referred
Q141: Of the following solvents which one does
Q142: What alkyl groups make up the following
Q143: The IR absorption frequencies of the C-H
Q145: Which compound is a primary amine with
Q146: For the following reaction sequence (it is
Q147: Carbon dioxide has a higher boiling point
Q148: What alkyl group is attached to the
Q149: The IR stretching frequency can be predicted