Essay
Explain why the compound shown is considered to be capable of being a soap (dissolving oily substances off of surfaces using water). 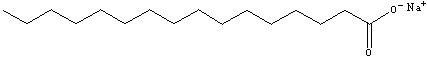
Correct Answer:

Verified
The compound contains a long hydrophobic...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q7: A tertiary carbon atom is present in
Q8: The number of unique open-chain structures corresponding
Q9: Which compound would have the lowest boiling
Q10: Even though methyl amine (CH<sub>3</sub>NH<sub>2</sub>)<sub> </sub>has a
Q11: A non-zero dipole moment is exhibited by:<br>A)SO<sub>2</sub><br>B)CO<sub>2</sub><br>C)CCl<sub>4</sub><br>D)BF<sub>3</sub><br>E)
Q13: n-Pentane has a higher boiling point than
Q14: What alkyl groups make up the following
Q15: Which molecule would have a dipole moment
Q16: In addition to a cycloalkane skeleton,testosterone also
Q17: Which alkane is predicted to have the