Multiple Choice
What is/are the product(s) of the following acid-base mechanism? 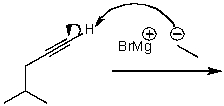
A) 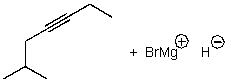
B) 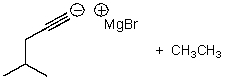
C) 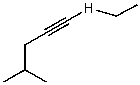
D) 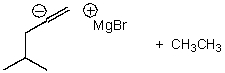
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: The basic species are arranged in decreasing
Q41: Adding sodium hydride,NaH,to water produces:<br>A)H<sub>2</sub> and NaOH(aq)<br>B)H<sup>-</sup>(aq)+
Q42: Which combination of reagents is effective in
Q43: What is the major product for the
Q44: Which combination of reagents is the
Q46: A substance that can donate a lone
Q47: Hydrogen atom(s)from which position(s)is (are)most likely to
Q48: Which is the weakest acid?<br>A)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CHFCO<sub>2</sub>H<br>B)CH<sub>3</sub>CHCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH<br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>SO<sub>3</sub>H<br>D)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH=CH<sub>2</sub><br>E)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>NH<sub>2</sub>
Q49: For the following acid-base reaction,which statement
Q50: Define a protic solvent.