Multiple Choice
Which of these phosphorus-based acids is diprotic?
A) 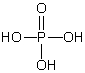
B) 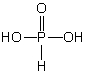
C) 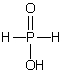
D) 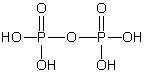
E) 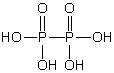
Correct Answer:

Verified
Correct Answer:
Verified
Q93: Which base would not effectively deprotonate phenol
Q94: What is/are the product(s)of the following acid-base
Q95: It is impossible to have pK<sub>a</sub> values
Q96: This species is a carbon-based Lewis acid:<br>A)CH<sub>4</sub><br>B)HCCl<sub>3</sub><br>C)CH<sub>3</sub><sup>+</sup><br>D):CH<sub>3</sub><sup>-</sup><br>E)·CH<sub>3</sub>
Q97: Rank the bold-faced hydrogens for the following
Q99: Adding sodium hydride to ethanol would produce:<br>A)CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub>
Q100: In the reaction,Na<sup>+</sup>NH<sub>2</sub><sup>-</sup> + CH<sub>3</sub>OH
Q101: According to Lewis theory,a base is a
Q102: Which of these is not a diprotic
Q103: The compounds ethane,ethene,and ethyne exhibit this order