For the Following Acid-Base Reaction,which Statement Is True Taking H into Consideration?
A)The Reaction Is an Exothermic Reaction
Multiple Choice
For the following acid-base reaction,which statement is true taking H into consideration? 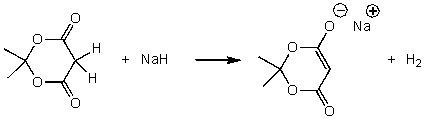
A) The reaction is an exothermic reaction and H is approximately zero.
B) The reaction is an endothermic reaction and H is negative.
C) The reaction is an endothermic reaction and H is positive.
D) The reaction is an exothermic reaction and H is negative.
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q113: What does the reaction between the following
Q114: Which combination of reagents is effective in
Q115: Consider the equilibrium <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="Consider the
Q116: What is/are the product(s)of the following acid-base
Q117: Which is the strongest acid?<br>A)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CHFCO<sub>2</sub>H<br>B)CH<sub>3</sub>CHBrCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CO<sub>2</sub>H<br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHClCH<sub>2</sub>CO<sub>2</sub>H<br>D)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHFCH<sub>2</sub>CO<sub>2</sub>H<br>E)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CHICH<sub>2</sub>CO<sub>2</sub>H
Q119: Bond polarization that takes place through space
Q120: Acetic acid dissociates most completely in:<br>A)CCl<sub>4</sub><br>B)Cl<sub>2</sub>C=CCl<sub>2</sub><br>C)H<sub>2</sub>O<br>D)(CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>O<br>E)the gas
Q121: What is the major product for the
Q122: Which sequence is the best one
Q123: For the equilibrium <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg" alt="For the