Multiple Choice
For the following acid/base reaction which statement is true taking S into consideration? 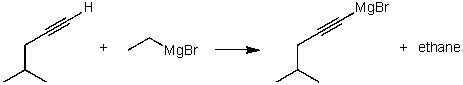
A) The reaction is an exothermic reaction and S is approximately zero.
B) The reaction is an endothermic reaction and S is negative.
C) The reaction is an exothermic reaction and S is negative.
D) The reaction is an exothermic reaction and S is positive.
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Which acid-base reaction would not take
Q22: When drawing mechanisms,chemists generally use curved arrows.The
Q23: Which reaction of these potential acids and
Q24: Which base would not effectively deprotonate acetylene?<br>A)LiOCH<sub>3</sub><br>B)CH<sub>3</sub>Li<br>C)CH<sub>3</sub>OCH<sub>2</sub>MgBr<br>D)KH<br>E)(CH<sub>3</sub>)<sub>2</sub>NLi
Q25: What is the conjugate base of ethanol?<br>A)CH<sub>3</sub>CH<sub>2</sub>O<sup>-</sup><br>B)CH<sub>3</sub>CH<sub>2</sub><sup>-</sup><br>C)CH<sub>3</sub>CH<sub>2</sub>OH<sub>2</sub><sup>+</sup><br>D)CH<sub>3</sub>CH<sub>3</sub><br>E)CH<sub>3</sub>OCH<sub>3</sub>
Q27: According to the Lewis definition,a base is
Q28: Which pair of species are both bases
Q29: Draw an arrow pushing mechanism to illustrate
Q30: Which base would most effectively deprotonate benzoic
Q31: Which reaction of these potential acids and