Multiple Choice
For the following acid/base reaction which statement is true taking S into consideration? 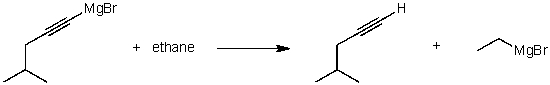
A) The reaction is an exothermic reaction and S is approximately zero.
B) The reaction is an endothermic reaction and S is negative.
C) The reaction is an exothermic reaction and S is negative.
D) The reaction is an exothermic reaction and S is positive.
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q55: The acidity constant,K<sub>a</sub>,differs from the equilibrium constant,K<sub>eq</sub>,for
Q56: Select the strongest base.<br>A)OH<sup>-</sup><br>B)RC <span class="ql-formula"
Q57: The reaction between which combination of substances
Q58: Which of these is not a Lewis
Q59: Write an equation to show the reaction
Q61: The compound aniline,C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub>,has weakly basic properties in
Q62: Which of these is not a true
Q63: Based on the position of the central
Q64: Why do water-insoluble carboxylic acids dissolve in
Q65: Rank the bold-faced hydrogens for the following