Multiple Choice
For the following acid / base reaction which statement is true taking H into consideration? 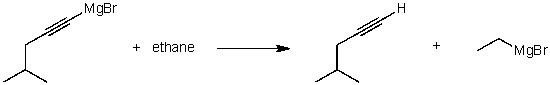
A) The reaction is an exothermic reaction and H is approximately zero.
B) The reaction is an endothermic reaction and H is negative.
C) The reaction is an exothermic reaction and H is negative.
D) The reaction is an exothermic reaction and H is positive.
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q100: In the reaction,Na<sup>+</sup>NH<sub>2</sub><sup>-</sup> + CH<sub>3</sub>OH
Q101: According to Lewis theory,a base is a
Q102: Which of these is not a diprotic
Q103: The compounds ethane,ethene,and ethyne exhibit this order
Q104: Rank the bold-faced hydrogens for the following
Q106: Which of the following organic compounds has
Q107: Rank the bold-faced hydrogens for the following
Q108: Rank the bold-faced hydrogens for the following
Q109: What reactants would give the following products?
Q110: Which of the following correctly lists