Multiple Choice
Consider the SN2 reaction of 2-iodopentane with CH3CO2- ion. 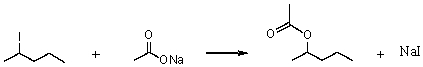
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and sodium acetate?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: When 5-bromo-1-pentanol is treated with sodium hydride
Q5: Select the rate law for the
Q6: S<sub>N</sub>1 reactions of the following type:
Q7: S<sub>N</sub>1 reactions of the type,Nu<sup>-</sup> +
Q8: Which ion is the strongest nucleophile in
Q10: Which of the following nucleophiles will react
Q11: Reaction of (R)-2-chloro-4-methylhexane with excess NaI in
Q12: Typically alkyl chlorides are slow in S<sub>N</sub>2
Q13: Considering the relative solvation of reactants
Q14: An endergonic S<sub>N</sub>2 reaction will have