Multiple Choice
Consider the SN2 reaction of 1-chloro-5-methylhexane with CN- ion. 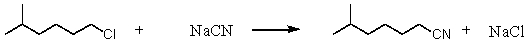
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 1-chloro-5-methylhexane and NaCN?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
Correct Answer:

Verified
Correct Answer:
Verified
Q68: Identify the leaving group in the following
Q69: Which of the following is not a
Q70: Which is the most reactive nucleophile in
Q71: Which alkyl halide would you expect to
Q72: An increase in the temperature at which
Q74: Select the potential energy diagram that represents
Q75: Which of the following nucleophiles will react
Q76: Which alkyl halide would be most reactive
Q77: Ambident nucleophiles are ones which can react
Q78: The substitution mechanism whose rate depends primarily