Multiple Choice
A compound with the molecular formula C4H10O gives a 1H NMR spectrum consisting only of a quartet centered at 3.5 and a triplet at 1.1.The most likely structure for the compound is:
A) 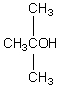
B) 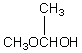
C) CH3CH2CH2CH2OH
D) CH3CH2OCH2CH3
E) 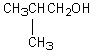
Correct Answer:

Verified
Correct Answer:
Verified
Q86: An unknown compound,C,has the formula C<sub>7</sub>H<sub>7</sub>Br.Elucidate the
Q87: What is the structure of the compound
Q88: An unknown compound,E,has the formula C<sub>6</sub>H<sub>12</sub>O.Elucidate the
Q89: What is the structure of the compound
Q90: Which proton(s)of the compound below would appear
Q92: What can be determined from the
Q93: What is the structure of the compound
Q94: Interpret the following <sup>13</sup>C/DEPT spectrum of a
Q95: Which one of the following best represents
Q96: What compound is used as the standard