Multiple Choice
A compound with the molecular formula C8H9BrO gave the following 1H NMR spectrum: triplet, 1.4
Quartet, 3.9
Multiplet, 7.0 (4H)
There was no evidence of an -OH band in the IR spectrum.A possible structure for the compound is: 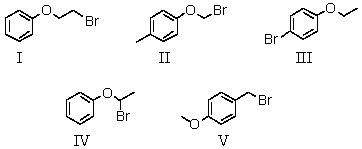
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Q66: The data below from the molecular
Q67: What is the structure of the compound
Q68: For the compound adamantine,how many different signals
Q69: In the structure shown,H<sub>a</sub> and H<sub>b</sub> are
Q70: Consider the expected <sup>1</sup>H NMR spectrum of
Q72: How many chemically distinct <sup>1</sup>H NMR signals
Q73: Predict the <sup>1</sup>H NMR spectrum of cis-1,4-cyclohexanediol
Q74: Which one of the following best represents
Q75: Which one of the following best represents
Q76: For the following compound how many different