Multiple Choice
For the following compound how many different signals would you see in the carbon NMR? (Assume that you can see them all.) 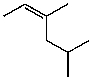
A) 4
B) 5
C) 6
D) 7
E) 8
Correct Answer:

Verified
Correct Answer:
Verified
Q46: The <sup>1</sup>H NMR spectrum of which of
Q47: For the following compound how many different
Q48: Briefly explain how you might distinguish between
Q49: Predict the splitting pattern you would observe
Q50: Consider the expected splitting of the C2
Q52: Examine the <sup>1</sup>H NMR spectrum of 1-nitropropane,shown
Q53: A prominent (M<sup>+</sup> <span class="ql-formula" data-value="\bullet"><span
Q54: A compound C<sub>5</sub>H<sub>11</sub>Cl which exhibits only two
Q55: Which is the base peak? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q56: What is the structure of the compound