Multiple Choice
Which compound below would give rise to 4 signals in the proton NMR spectrum and 4 signals in the carbon NMR spectrum? (Assume you can separate and see all peaks.) 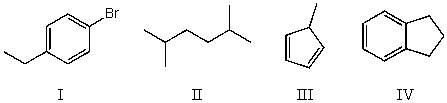
A) I
B) II
C) III
D) IV
E) More than one of the above.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: How many <sup>13</sup>C signals would 1,3-dichlorobenzene give?
Q24: For the following compound how many different
Q25: What is the molecular formula of
Q26: What is the structure of the compound
Q27: What is the structure of the compound
Q29: If all the protons of 1-fluoropentane could
Q30: A compound with the molecular formula
Q31: What is the structure of the compound
Q32: A compound that would show two triplets
Q33: Predict the <sup>1</sup>H NMR spectrum of 2-chloroethanal,CH<sub>2</sub>ClCHO.