Multiple Choice
Which compound below would not give rise to 4 signals in the proton NMR spectrum and 3 signals in the carbon NMR spectrum? (Assume you can separate and see all peaks.) 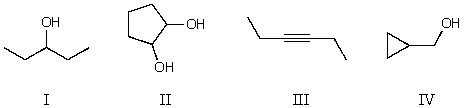
A) I
B) II
C) III
D) IV
E) All of these choices fit the criteria.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For the compound dimedone,how many different signals
Q2: All of the hydrogen atoms in a
Q3: An unknown compound,L,has the formula C<sub>5</sub>H<sub>10</sub>O<sub>2</sub>.Elucidate the
Q5: A compound with the molecular formula
Q6: The broadband proton-decoupled <sup>13</sup>C NMR spectrum of
Q7: Which compound below would give rise to
Q8: Which is the likely molecular ion
Q9: What is the structure of the compound
Q10: What is the structure of the compound
Q11: Which one of the following best represents