Multiple Choice
Which compound below would give rise to 5 signals in the proton NMR spectrum and 7 signals in the carbon NMR spectrum? (Assume you can separate and see all peaks.) 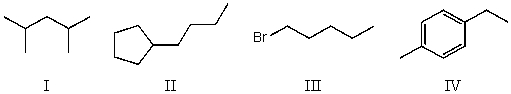
A) I
B) II
C) III
D) IV
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q52: Examine the <sup>1</sup>H NMR spectrum of 1-nitropropane,shown
Q53: A prominent (M<sup>+</sup> <span class="ql-formula" data-value="\bullet"><span
Q54: A compound C<sub>5</sub>H<sub>11</sub>Cl which exhibits only two
Q55: Which is the base peak? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5902/.jpg"
Q56: What is the structure of the compound
Q58: What is the structure of the compound
Q59: Which one of the following best represents
Q60: Consider the expected splitting of signal "b"
Q61: How many signals would you expect to
Q62: A compound C<sub>5</sub>H<sub>10</sub>O gave the following