Multiple Choice
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H18O2 and characteristic 13C-NMR peaks at 11.3,21.6,25.3,49.4,67.1,and 175.5 ppm? Relative integration is shown. 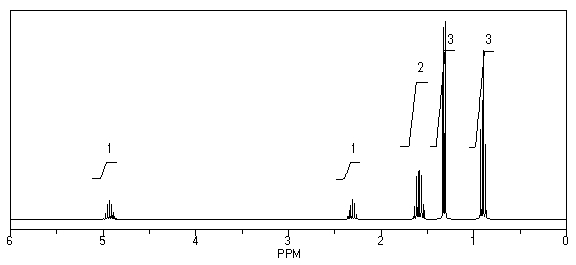
A) 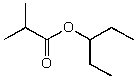
B) 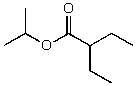
C) 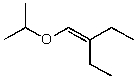
D) 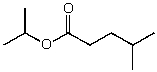
E) 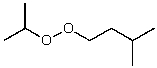
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Predict the <sup>1</sup>H NMR spectrum of 2-chloroethanal,CH<sub>2</sub>ClCHO.
Q34: Briefly explain how you might distinguish between
Q35: The predicted ratio of the relative peak
Q36: Compounds that contain a benzylic carbon will
Q37: In the structure shown,H<sub>a</sub> and H<sub>b</sub> are
Q39: For the compound dimedone,how many different signals
Q40: Which one of the following best represents
Q41: Hydrogen that are diastereotopic will show different
Q42: Consider the expected splitting of signal "b"
Q43: Predict the <sup>1</sup>H NMR spectrum of diethoxymethane,CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub>.