Multiple Choice
The accompanying diagram,which describes the fate of the intermediate in a reversible reaction,implies that: 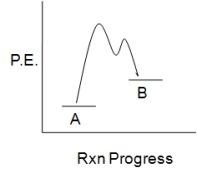
A) the less stable product forms more rapidly.
B) the more stable product forms more rapidly.
C) product B will predominate at equilibrium.
D) the intermediate has a short lifetime.
E) No conclusions can be drawn as to either reaction rate or product stability.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Which reaction would produce the following compound?
Q12: A thermodynamically-controlled reaction will yield predominantly:<br>A)the more/most
Q13: Which carbocation would be least stable? <img
Q14: 1,3-Butadiene has how many electrons in
Q15: The allyl cation has how many
Q17: The accompanying diagram,which describes the fate of
Q18: The allyl radical has how many
Q19: Which would be the best synthesis of
Q20: The substituent R on the bicyclic compound
Q21: Ignoring stereochemistry,the 1:1 reaction of bromine