Essay
Which diene and dienophile would you use to prepare the following molecule using a Diels-Alder cycloaddition reaction: 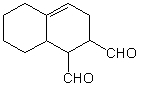
Correct Answer:

Verified
Correct Answer:
Verified
Q152: Which reagent would convert 1,3-octadiene into 3-octen-2-ol?<br>A)KMnO<sub>4</sub>/<sup>-</sup>OH<br>B)OsO<sub>4</sub><br>C)H<sub>2</sub>O<sub>2</sub>,then
Q153: Which carbocation would be most stable?<br>A) <img
Q154: The HOMO of the allylic cation has
Q155: A thermodynamically-controlled reaction will yield predominantly: _.
Q156: Which carbocation would be most stable? <img
Q158: During Diels-Alder reactions,when two stereoisomer products,exo and
Q159: Which of these dienes can undergo the
Q160: The structure below is an intermediate along
Q161: Select the structure(s)of the conjugated diene(s). <img
Q162: Show the steps and reagents necessary to