Multiple Choice
Consider the resonance forms shown for the arenium ionformed from the bromination of acetanilide.Which resonance form contributes most to the overall resonance hybrid? 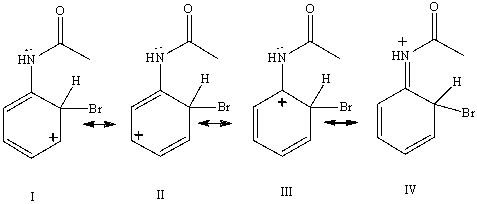
A) I
B) II
C) III
D) IV
E) All contribute equally.
Correct Answer:

Verified
Correct Answer:
Verified
Q110: Which of the following is not an
Q111: What is the chief product of the
Q112: Which of the following structures contribute(s)to the
Q113: The major product(s)of the following reaction, <img
Q114: When two different groups are present on
Q116: S<sub>N</sub>1 solvolysis of C<sub>6</sub>H<sub>5</sub>CH=CHCH<sub>2</sub>Cl in water produces:<br>A)C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>C(OH)=CH<sub>2</sub><br>B)C<sub>6</sub>H<sub>5</sub>CH=CHCH<sub>2</sub>OH<br>C)C<sub>6</sub>H<sub>5</sub>CHOHCH=CH<sub>2</sub><br>D)A
Q117: Draw a mechanism that explains the formation
Q118: The electrophilic bromination or chlorination of benzene
Q119: Acid-catalyzed hydration of 1-phenyl-1-pentene gives 1-phenyl-1-pentanol almost
Q120: What would you expect to be the