Multiple Choice
A compound has the molecular formula C8H14O4.Its IR spectrum shows a strong absorption band near 1740 cm-1.Its 1H NMR spectrum consists of: triplet, 1.3
Singlet, 2.6
Quartet, 4.2
The most likely structure for the compound is: 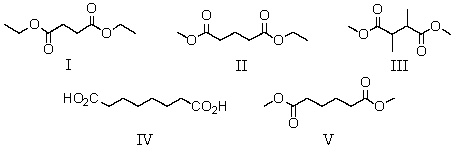
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q158: What is the product of the reaction
Q159: Predict the final product likely to be
Q160: What is the reactant of the following
Q161: Which of the following would be the
Q162: Which compound would be expected to have
Q164: What would be the final product,F,of the
Q165: Given a mixture of benzyl alcohol,phenol,and benzoic
Q166: An acid-catalyzed esterification (a reaction between a
Q167: Which of the following is the best
Q168: Which of the following acids would be