Multiple Choice
A compound with the molecular formula C5H10O2 gave the following 1H NMR spectrum: triplet, 0.90
Multiplet, 1.60
Singlet, 1.95
Triplet, 3.95
The IR spectrum showed a strong absorption band near 1740 cm-1.The most likely structure for the compound is:
A) 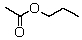
B) 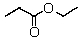
C) 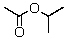
D) 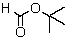
E) 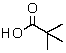
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Alkaline hydrolysis of an ester involves initial
Q4: What is the final product of this
Q5: What is the final product of this
Q6: Which of these combinations will not produce
Q7: Choose the reagent(s)that would bring about
Q9: What is the product of this reaction?
Q10: Identify the product(s)of the following reaction. <img
Q11: What would be the final product? <img
Q12: What is the expected product,A,of the following
Q13: Which of the following would be the