Multiple Choice
What synthetic strategy would accomplish the following transformation? 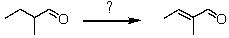
A) i) Br2,H3O+; ii) NaOC2H5,C2H5OH,heat
B) i) Cl2,FeCl3; ii) NaOC2H5,C2H5OH,heat
C) i) HCN; ii) H3O+,heat
D) i) Br2,h ; ii) (CH3) 3COK,(CH3) 3COH,heat
E) None of these choices.
Correct Answer:

Verified
Correct Answer:
Verified
Q129: What would be the major product of
Q130: Which compound would be formed when 2-methylbutanal
Q131: Considering only the highlighted hydrogens,list the following
Q132: What would be the major product of
Q133: What is the final product of the
Q135: Which of the following represent keto-enol tautomers?<br>A)
Q136: Which of these compounds would exist primarily
Q137: What product is finally formed when the
Q138: Diastereomers that differ in configuration at only
Q139: Which reagent would best serve as the