Multiple Choice
The free energy of activation, ΔG 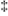 , is defined as:
, is defined as:
A) The average free energy of the product formed.
B) The rate of a chemical reaction in relationship to the concentration of reactant molecules.
C) The energy required to raise the average energy of one mole of reactant to the transition state energy.
D) The amount of energy released by a spontaneous reaction.
E) The lowest point on a free energy diagram.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: A plot of 1/V vs. 1/[S] for
Q18: In the enzyme catalyzed reaction sequence below,
Q19: All are true for k<sub>cat</sub> EXCEPT :<br>A)
Q20: The International Units of an enzyme are
Q21: All are characteristics of the enzyme hexokinase
Q23: Which statement is correct about the Michaelis-Menten
Q24: In the reaction mechanism below, _ are
Q25: For an enzyme-catalyzed reaction, the initial velocity
Q26: Identify the type of reaction that would
Q27: If an enzyme has a V<sub>max</sub> of