Multiple Choice
The carbon tetrachloride molecule,CC 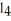 ,is ________.
,is ________.
A) a polar molecule with polar bonds
B) a nonpolar molecule with polar bonds
C) a nonpolar molecule with nonpolar bonds
D) a polar molecule with nonpolar bonds
E) a polar molecule with ionic bonds
Correct Answer:

Verified
Correct Answer:
Verified
Q59: The carbon tetrachloride molecule,CC <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The
Q60: What is the correct formula for iron(III)sulfide?<br>A)
Q61: In a covalently bonded molecule,the number of
Q62: H2O has a double bond.
Q63: The name of the compound AlCl3 is
Q65: The molecule CO2 is linear.
Q66: What is the correct formula for the
Q67: A nonpolar molecule can have polar bonds.
Q68: A polar covalent bond is found in
Q69: When Al3+ and Br- combine,the formula of