Multiple Choice
The number of calories needed to raise the temperature of 32 g of water from 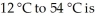 ________.
________.
A) 380 cal
B) 1.3 cal
C) 1300 cal
D) 1700 cal
E) 0.76 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: The specific heat of a substance is
Q50: Iron rusting is an example of a
Q51: Condensation occurs when a liquid is converted
Q52: A temperature of 41 °F is the
Q53: The heat of fusion for water is
Q55: Which of the following is not a
Q56: Which of the following is a physical
Q57: A bungee jumper just before she leaps,has
Q58: A temperature of 20°C is equivalent to
Q59: The heat of fusion is greater than