Multiple Choice
If the heat of fusion for water is 80.cal/g,how many calories are needed to melt 45.0 g of ice at 0 °C?
A) 3.6 cal
B) 3.6 × 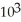 cal
cal
C) 1.8 cal
D) 80.cal
E) 0.56 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: Which of the following is a property
Q43: Which of the following would not be
Q44: A compound only contains one type of
Q45: Steam at 100 °C contains the same
Q46: Water freezes at 100 °C.
Q48: Which of the following quantities is not
Q49: The specific heat of a substance is
Q50: Iron rusting is an example of a
Q51: Condensation occurs when a liquid is converted
Q52: A temperature of 41 °F is the