Multiple Choice
Which of the following is correctly identified?
A) 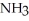 ,strong acid
,strong acid
B) NaOH,strong base
C) HCl,weak acid
D) 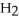
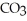 ,strong acid
,strong acid
E) Ca 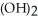 ,weak base
,weak base
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: 25.0 mL of 0.212 M NaOH is
Q20: The [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The [
Q21: Which of the following is a buffer
Q22: A buffer is a solution that tends
Q23: When an acid reacts with a metal
Q25: What is the pH of a solution
Q26: A system is at equilibrium when the
Q27: In a neutralization reaction _.<br>A)two acids react
Q29: If a solution has <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="If
Q31: HF is a strong acid.