Multiple Choice
According to the Brønsted-Lowry definition,________.
A) an acid is a proton acceptor
B) a base produces 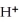 ions in aqueous solutions
ions in aqueous solutions
C) a base is a proton donor
D) a base is a proton acceptor
E) an acid acts as the solvent
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: KOH is a strong base.
Q10: Alkalosis is the blood condition in which
Q11: The name of NaOH is sodium hydroxide.
Q12: HBr is a strong acid.
Q13: A 10.0 mL of 0.121 M H2SO4
Q15: Strong acids react with Zn metal to
Q16: The conjugate acid of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The
Q17: What is the name of the medical
Q18: If the carbon dioxide level in the
Q19: 25.0 mL of 0.212 M NaOH is