Multiple Choice
According to LeChâtelier's principle,predict whether adding 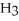
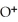 causes the system to shift in the direction of reactants,products,or no change. N
causes the system to shift in the direction of reactants,products,or no change. N 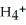 (aq) +
(aq) + 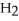 O(l) ⇌ N
O(l) ⇌ N 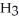 (aq) +
(aq) + 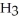
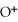 (aq)
(aq)
A) reactants
B) products
C) no change
Correct Answer:

Verified
Correct Answer:
Verified
Q35: When more reactant is added to a
Q36: Identify the Brønsted-Lowry acid in the following
Q37: An aqueous solution with <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="An
Q38: What is the [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="What
Q39: For many reactions of acids with bases,the
Q41: Which of the following could be a
Q42: An acidic solution has a pH less
Q43: Which of the following is the strongest
Q44: When a system is at equilibrium,all the
Q45: The function of a buffer is to