Multiple Choice
Which of the following is a neutralization reaction?
A) KCl (aq) + 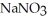 (aq) →
(aq) → 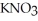 (aq) + NaCl(aq)
(aq) + NaCl(aq)
B) 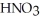 (aq) + KOH(aq) →
(aq) + KOH(aq) → 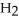 O (l) +
O (l) + 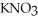 (aq)
(aq)
C) 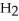 O(l) +
O(l) + 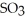 (g) →
(g) → 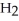
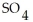 (aq)
(aq)
D) 4Na(s) + 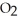 (g) → 2
(g) → 2 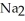 O(s)
O(s)
E) 2 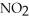 (g) → 2NO(g) +
(g) → 2NO(g) + 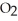 (g)
(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q45: The function of a buffer is to
Q46: Which one of the following is characteristic
Q47: According to the Arrhenius concept,if <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg"
Q48: The conjugate base of HF is F-.
Q49: In any aqueous solution, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="In
Q51: The [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The [
Q52: According to LeChâtelier's principle,predict whether adding H2O
Q53: The [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="The [
Q54: Acids turn litmus blue.
Q55: What is the [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6909/.jpg" alt="What