Multiple Choice
The correct formula for the ionic compound containing Al3+ and PO 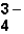 would be _____.
would be _____.
A) Al3(PO4) 3
B) AlPO4
C) Al3(PO4) 2
D) Al2(PO4) 3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The lattice site in a crystal is<br>A)the
Q1: When magnesium forms an ion,it would be
Q17: In describing the strength of interparticle forces,
Q34: When an ionic compound forms between sodium
Q35: The outer shell of a noble gas
Q41: The anion in an ionic compound whose
Q48: Which of the following Lewis structures depicts
Q68: What would be the proper name for
Q81: A covalent bond results when<br>A)one atom gives
Q82: Both bonding and non-bonding electron pairs can