Multiple Choice
Which of the following correctly represents the polarity of the bond?
A) 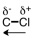
B) 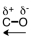
C) 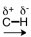
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Na<sup>+</sup> and F<sup>-</sup>are isoelectronic.
Q14: If the electronegativity difference between A and
Q21: The ionic compound that forms between magnesium
Q36: Which of the following would cause a
Q39: Dispersion forces are among the strongest interparticle
Q42: Which of the following metals would not
Q48: The correct formula for ammonium sulfate is
Q52: Carbon dioxide is a linear molecule.
Q58: H<sub>2</sub>O is a binary compound.
Q60: During the formation of an ionic bond