Essay
At a temperature of 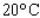 , the volume V (in liters) of 1.33 g of
, the volume V (in liters) of 1.33 g of 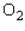 is related to its pressure p (in atmospheres) by the formula
is related to its pressure p (in atmospheres) by the formula 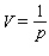 .
.
What is the average rate of change of V with respect to p as p increases from 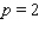 to
to 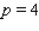 ? What is the rate of change of V with respect to p when
? What is the rate of change of V with respect to p when 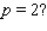
Correct Answer:

Verified
Correct Answer:
Verified
Q156: Let <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6026/.jpg" alt="Let .
Q157: The following graph shows the ratio of
Q158: Let <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6026/.jpg" alt="Let ,
Q159: Use the graph of the function f
Q160: Find the limit, if it exists. Otherwise,
Q162: A division of Chapman Corporation manufactures a
Q163: Find the slope of the tangent line
Q164: Find the values of x for which
Q165: Determine all values of x at which
Q166: Use the intermediate value theorem to find