Multiple Choice
Indicate which metal requires the shortest wavelength photons to eject photoelectrons based on the following data and graph.Work functions are in J 10-19. 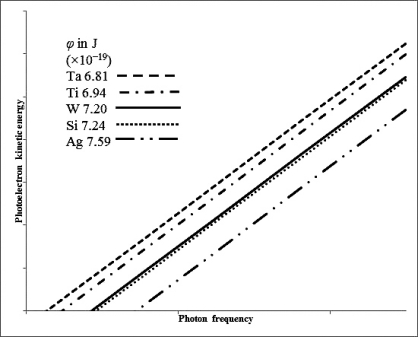
A) Ta
B) Ti
C) W
D) Si
E) Ag
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: In quantum mechanics, an atomic orbital _.<br>A)provides
Q32: Which of the following statements regarding orbitals
Q116: Which arrangement is correct for increasing atomic
Q139: Which of the transitions in the hydrogen
Q140: Transition metal ions typically lose their outermost
Q141: Which of the following elements has the
Q143: A shell consists of all _<br>A)electrons with
Q145: What information do boundary-surface representations of orbitals
Q146: Which of the following statements is NOT
Q147: Which of the following represents an s