Multiple Choice
The energy change for an electronic transition in a one-electron atom or ion (H,He+,Li2+,etc.) from ninitial to nfinal is given by 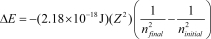 ,where Z is the atomic number. How much energy is required to ionize a ground-state He+ ion (nfinal = ) ?
,where Z is the atomic number. How much energy is required to ionize a ground-state He+ ion (nfinal = ) ?
A) 2.18 10-18 J
B) 4.36 10-18 J
C) 8.72 10-18 J
D) 1.09 10-18 J
E) 5.45 10-19 J
Correct Answer:

Verified
Correct Answer:
Verified
Q15: The energy change for an electronic transition
Q18: What is the approximate uncertainty in
Q19: What is the change in energy
Q21: How many times longer is the
Q22: What is the shortest wavelength of light
Q23: Which of the following is a possible
Q24: The following orbital diagrams show the n
Q25: Suppose a bar code scanner emits
Q80: Which of the following will lead to
Q140: Which statement about electromagnetic radiation is NOT