Multiple Choice
Which set of quantum numbers could be correct for the highest energy electron in lead,Pb?
A) n = 5,  = 1,m
= 1,m  = 0,ms = -
= 0,ms = - 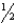
B) n = 5,  = 2,m
= 2,m  = -1,ms = +
= -1,ms = + 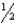
C) n = 6,  = 1,m
= 1,m  = 0,ms = -
= 0,ms = - 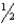
D) n = 6,  = 1,m
= 1,m  = 2,ms = +
= 2,ms = + 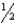
E) n = 6,i = 2,m  = 0,ms = -
= 0,ms = - 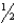
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Indicate which of the following photons can
Q32: Which of the following statements regarding orbitals
Q47: Which of the following is the ground-state
Q64: Astronomers have detected hydrogen atoms in interstellar
Q143: A shell consists of all _<br>A)electrons with
Q145: What information do boundary-surface representations of orbitals
Q146: Which of the following statements is NOT
Q147: Which of the following represents an s
Q150: What is the approximate total uncertainty
Q153: Which of the following is the correct