Multiple Choice
Consider the molecule and the following hybridization choices: 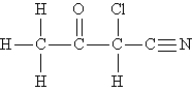
-What is the hybridization of the nitrogen atom?
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: Consider the skeletal structure shown below:<br>N-C-C-N<br>Draw the
Q54: The hybridization of a molecule is measured
Q55: The hybridization of the central atom in
Q56: When a carbon atom has sp<sup>3</sup>
Q57: A species has the following MO
Q59: For which of the following diatomic molecules
Q60: _ is the difference between the number
Q61: The hybridization of the lead atom in
Q62: The bond order for CN<sup>-</sup> is 2.
Q63: What is the bond order of C<sub>2</sub><sup>+</sup>?<br>A)0<br>B)